homebrandcrumpet
Newbie
Beginner's error! I'm getting my chemicals together for I could only find sodium thiosulfate from camera stores in my region, and I couldn't find any information on how to substitute this for sulphate so I was stumped. I eventually managed to find some sodium sulphite from a local preservatives manufacturer, however when it arrived the ingredients were listed as:
Preservative 221 (CONTAINS: 49% SULPHUR DIOXIDE, SODIUM SULPHITE).
This wasn't described on their webstore, only on the label when the bag arrived. I'm guessing I've ordered food-grade sodium sulphite instead of photochemical grade? Can this be safely substituted or should I send it back and keep seraching? Any help with these substitutions would also be greatly appreciated.
Preservative 221 (CONTAINS: 49% SULPHUR DIOXIDE, SODIUM SULPHITE).
This wasn't described on their webstore, only on the label when the bag arrived. I'm guessing I've ordered food-grade sodium sulphite instead of photochemical grade? Can this be safely substituted or should I send it back and keep seraching? Any help with these substitutions would also be greatly appreciated.
x-ray
Mentor
Send it back. Sulphur Dioxide is a toxic gas you don’t want to mess with. I guess what you have is a solid form but mixed with the wrong agent you could release it. It’s been fifty five years since college but I have a degree in chemistry and microbiology and know SO2 is very toxic.
Check Chensavers for what you need. Chemsavers Products - Chemsavers, Inc.
I buy diethyl ether and potassium cyanide from them for wet plate photography. You should be able to get it from Bostic & Sullivan in Santa Fe. They’re a photo chemical supplier for alternative printing processed.

 www.bostick-sullivan.com
www.bostick-sullivan.com
Probably Chemsavers will be cheaper but not sure. They also sell through EBay too.
I’m guessing what you wound up with was formulated for wine makers or brewing beer. Sodium sulfite and I believe sulfur dioxide are used to to sterilize equipment and sodium sulfite preserves food and wine by retarding oxidation. In photo chemicals it accelerates development, reduction, and retards oxidation.
Over 60 years of mixing and using photo chemicals I’ve absorbed enough sodium sulfite in my system to sensitize receptors in my heart. I have a bad reaction to foods containing sulfites especially things like MSG flavor enhancers and sulfites for preserving wine and foods. The result is extreme heart arrhythmia. There have been several instances with people like myself that have sensitivities to sulfites to induce fatal heart attacks. I unfortunately have to be very careful in what wines I drink and the quantity. Several countries like Italy, France and Argentina restrict the addition of sodium sulfite so I’m forced to drink the great wines they produce. Darn 🍷 😁.
Check Chensavers for what you need. Chemsavers Products - Chemsavers, Inc.
I buy diethyl ether and potassium cyanide from them for wet plate photography. You should be able to get it from Bostic & Sullivan in Santa Fe. They’re a photo chemical supplier for alternative printing processed.

35 ml Traditional Platinum & Palladium Combo Kit
Get all the supplies necessary for traditional Platinum and Palladium printing. Make up to 35 8″x10″ prints using our pre-mixed chemistry. By combining Platinum and Palladium, you get the smoothness of tone that palladium produces, while benefiting from the coolness and contrast boost Platinum...
Probably Chemsavers will be cheaper but not sure. They also sell through EBay too.
I’m guessing what you wound up with was formulated for wine makers or brewing beer. Sodium sulfite and I believe sulfur dioxide are used to to sterilize equipment and sodium sulfite preserves food and wine by retarding oxidation. In photo chemicals it accelerates development, reduction, and retards oxidation.
Over 60 years of mixing and using photo chemicals I’ve absorbed enough sodium sulfite in my system to sensitize receptors in my heart. I have a bad reaction to foods containing sulfites especially things like MSG flavor enhancers and sulfites for preserving wine and foods. The result is extreme heart arrhythmia. There have been several instances with people like myself that have sensitivities to sulfites to induce fatal heart attacks. I unfortunately have to be very careful in what wines I drink and the quantity. Several countries like Italy, France and Argentina restrict the addition of sodium sulfite so I’m forced to drink the great wines they produce. Darn 🍷 😁.
Last edited:
homebrandcrumpet
Newbie
Forewarned is forearmed! My partner is very sensitive to sulphites - great motivation to not skimp out on gloves and ventilation in my would-be darkroom. Are there alternatives to sulhpite-based B&W developers that you've gravitated towards? Thanks so much for the brilliant information.
Does anyone know whether sodium thiosuplhate works as a 1:1 substitute for sodium sulphite, or do you need to adjust the ratios? It seems to be more common than sodium sulphate in Australia, but nobody selling it offers any guidance on how to use it.
Does anyone know whether sodium thiosuplhate works as a 1:1 substitute for sodium sulphite, or do you need to adjust the ratios? It seems to be more common than sodium sulphate in Australia, but nobody selling it offers any guidance on how to use it.
sojournerphoto
Mentor
Thiosullhates are used as fixer - they dissolve the unreduced (i.e. undeveloped silver halide crystals). Adding it to developer may leave you with no or only a limited image, so not a substitute for sulphite (not withstanding one-bath chemicals).Forewarned is forearmed! My partner is very sensitive to sulphites - great motivation to not skimp out on gloves and ventilation in my would-be darkroom. Are there alternatives to sulhpite-based B&W developers that you've gravitated towards? Thanks so much for the brilliant information.
Does anyone know whether sodium thiosuplhate works as a 1:1 substitute for sodium sulphite, or do you need to adjust the ratios? It seems to be more common than sodium sulphate in Australia, but nobody selling it offers any guidance on how to use it.
You can make developers without sodium sulphite. You need the developing agent and an alkali. One such is PC-TEA, but there are others.
Sulphite is often used to help manage the appearance of grain, as well as acting as a preservative for the developing agents and in some developers providing the alkali (D23 for example). There are alternatives.
Mike
sojournerphoto
Mentor
Here’s a similar developer
Gainer minimalist developer
And one source for the pc-tea recipe
PC-TEA
Both these are based on Vitamin C with phenidone. An alternative uses Vit C and coffee - search for the caffenol cookbook or caffenol c-m and c-h
Gainer minimalist developer
And one source for the pc-tea recipe
PC-TEA
Both these are based on Vitamin C with phenidone. An alternative uses Vit C and coffee - search for the caffenol cookbook or caffenol c-m and c-h
Benjamin Marks
Mentor
There is a learning curve for all this stuff, and you are on your way. +1 to the above: thiosulfates (sodium or ammonium) are are fixing agents. Sodium sulfite is an anti-oxidant that allows developer more working life. It also has the grain effects noted above. From a photographic perspective, the two have nothing to do with one another other than both contain sodium.
I'm going to suggest that you do some research into tried and true photographic formulas (e.g. from here: Photographers Formulary) and that you not deviate from the recipies based on random advice from the Internet. While generally benign, some chemicals absolutely should not be mixed together (particularly anything containing KCN, potassium cyanide and anything, if you don't know what you are doing) and this is a "words do matter" sort of deal.
Sometimes you can make one chemical from another, e.g. sodium carbonate from sodium bicarbonate (baking soda), but there will be plenty of chemicals that look related because they share a chemical component that simply are not.
See if you can find a used copy of Steve Anchell's Darkroom Cookboook. Here, for instance: Darkroom Cookbook. That should set you on your way.
Good luck! Mixing your own film and paper developers can give you an unparalled amount of control over your development process. If you develop a lot of film, it can be cost effective too. And you get to learn cool stuff.
I'm going to suggest that you do some research into tried and true photographic formulas (e.g. from here: Photographers Formulary) and that you not deviate from the recipies based on random advice from the Internet. While generally benign, some chemicals absolutely should not be mixed together (particularly anything containing KCN, potassium cyanide and anything, if you don't know what you are doing) and this is a "words do matter" sort of deal.
Sometimes you can make one chemical from another, e.g. sodium carbonate from sodium bicarbonate (baking soda), but there will be plenty of chemicals that look related because they share a chemical component that simply are not.
See if you can find a used copy of Steve Anchell's Darkroom Cookboook. Here, for instance: Darkroom Cookbook. That should set you on your way.
Good luck! Mixing your own film and paper developers can give you an unparalled amount of control over your development process. If you develop a lot of film, it can be cost effective too. And you get to learn cool stuff.
titrisol
Bottom Feeder
That is just a measure of the purity of the sulfite.Beginner's error! I'm getting my chemicals together for I could only find sodium thiosulfate from camera stores in my region, and I couldn't find any information on how to substitute this for sulphate so I was stumped. I eventually managed to find some sodium sulphite from a local preservatives manufacturer, however when it arrived the ingredients were listed as:
Preservative 221 (CONTAINS: 49% SULPHUR DIOXIDE, SODIUM SULPHITE).
This wasn't described on their webstore, only on the label when the bag arrived. I'm guessing I've ordered food-grade sodium sulphite instead of photochemical grade? Can this be safely substituted or should I send it back and keep seraching? Any help with these substitutions would also be greatly appreciated.
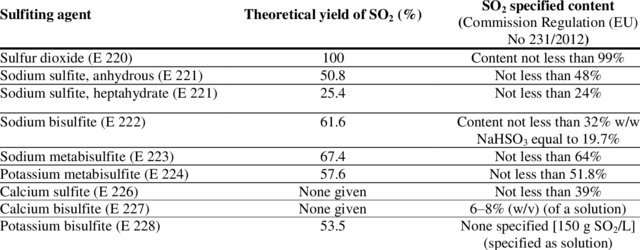
221 is supposed to be good purity Sulfite (90%+)
The equivalent yield of SO2 is used as a way to measure purity and is used in specs and in the European deifnitions
49% SO2 is about 96-99% Sulfite and thus perfectly usable
Check this spec for instance
titrisol
Bottom Feeder
From
Scientific Opinion on the re-evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228) as food additives
April 2016
EFSA Journal 14(4)
DOI: 10.2903/j.efsa.2016.4438
Scientific Opinion on the re-evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228) as food additives
April 2016
EFSA Journal 14(4)
DOI: 10.2903/j.efsa.2016.4438
Attachments
x-ray
Mentor
Sodium thiosulfate is the primary ingredient in fixer. It will clear the silver halide off your film.Forewarned is forearmed! My partner is very sensitive to sulphites - great motivation to not skimp out on gloves and ventilation in my would-be darkroom. Are there alternatives to sulhpite-based B&W developers that you've gravitated towards? Thanks so much for the brilliant information.
Does anyone know whether sodium thiosuplhate works as a 1:1 substitute for sodium sulphite, or do you need to adjust the ratios? It seems to be more common than sodium sulphate in Australia, but nobody selling it offers any guidance on how to use it.
I’m not aware of any other chemical that will substitute.
skahde
V for Victory!
If you are sensitive to sulfites go digital or let someone else do the darkroom-work and go hybrid. Trying to circumvent it means to many dirty compromises.
Muggins
Junk magnet
Thank you, Titrisol - I was looking that as a (currently somewhat brain dead) biochemist and wondering why it hadn't horribly gassed the OPer!That is just a measure of the purity of the sulfite.
221 is supposed to be good purity Sulfite (90%+)
The equivalent yield of SO2 is used as a way to measure purity and is used in specs and in the European deifnitions
Freakscene
Obscure member
This is absolutely correct. This is a food grade product and this is the product spec.That is just a measure of the purity of the sulfite.
221 is supposed to be good purity Sulfite (90%+)
The equivalent yield of SO2 is used as a way to measure purity and is used in specs and in the European deifnitions
49% SO2 is about 96-99% Sulfite and thus perfectly usable
Check this spec for instance
Which chemical do you need? You use sulfate, sulfite and thiosulfate in your post. Thiosulfate is a reducing agent and fixes photographic silver salts. I strongly recommend if you use any modern products that you use ammonium thiosulfate not sodium thiosulfate because it fixes silver iodide much more effectively. Having said that, you can get sodium thiosulfate from swimming pool supplies retailers - it is usually called ‘chlorine neutraliser’ or similar, like this:
Sulfite is an antioxidant and is used in developers and helps remove thiosulfate from fibre base papers. Sulfate is mostly used as a bulking agent in detergents and in paper making and as a drying agent in chemical synthesis. Its use is limited in photography.
Marty
Last edited:
wlewisiii
Just another hotel clerk
A package of generic D-76, a package of Diafine, 2 gallon packages of generic odorless fixer, and several 1 gallon jugs from Freestyle and your set for quite a while.
Unless there is a really specific reason I'm missing, (and I did go through a "Mix my own" phase. It was short, ugly and expensive) these will do for 99% of photography.
Unless there is a really specific reason I'm missing, (and I did go through a "Mix my own" phase. It was short, ugly and expensive) these will do for 99% of photography.
nikon_sam
Shooter of Film...
Dogman
Mentor
I bought a 25lb tub of sodium sulfite from the Chemistry Store online when I still shot film and had a darkroom. It was cheap at the time so I figured the quantity was more than enough. It was. I still had more than 2/3 of that tub full of the stuff when I shut down my darkroom. I checked to see how to dispose of it and was told it was not hazardous so it could go to the local landfill.
homebrandcrumpet
Newbie
Glad I didn't go ahead and order it as a substitute! It's unfortunate that neither of the two film developing supply distributors in my region give any explanation or warnings in their product descriptions to avoid potential confusion for beginners - to a novice sodium thiosulfate sounds like it could be interchangeable derivative of sodium sulfite and maybe do the same thing if you can work out the proper ratio. But as you point out — they do completely different things.Thiosullhates are used as fixer - they dissolve the unreduced (i.e. undeveloped silver halide crystals). Adding it to developer may leave you with no or only a limited image, so not a substitute for sulphite (not withstanding one-bath chemicals).
You can make developers without sodium sulphite. You need the developing agent and an alkali. One such is PC-TEA, but there are others.
Sulphite is often used to help manage the appearance of grain, as well as acting as a preservative for the developing agents and in some developers providing the alkali (D23 for example). There are alternatives.
Mike
Hopefully this thread will show up in search results and save some new developers from wasting some rolls. Can you recommend any books that actually help to explain what these chemicals are doing in different film development processes?
Good to know I've internalised at least two lessons from my high school chemistry!
- Follow the recipe to the letter; and,
- If in doubt — don't **** around and find out!
homebrandcrumpet
Newbie
Thank you so much for the recommendation, I'm definitely ordering a copy!There is a learning curve for all this stuff, and you are on your way. +1 to the above: thiosulfates (sodium or ammonium) are are fixing agents. Sodium sulfite is an anti-oxidant that allows developer more working life. It also has the grain effects noted above. From a photographic perspective, the two have nothing to do with one another other than both contain sodium.
I'm going to suggest that you do some research into tried and true photographic formulas (e.g. from here: Photographers Formulary) and that you not deviate from the recipies based on random advice from the Internet. While generally benign, some chemicals absolutely should not be mixed together (particularly anything containing KCN, potassium cyanide and anything, if you don't know what you are doing) and this is a "words do matter" sort of deal.
Sometimes you can make one chemical from another, e.g. sodium carbonate from sodium bicarbonate (baking soda), but there will be plenty of chemicals that look related because they share a chemical component that simply are not.
See if you can find a used copy of Steve Anchell's Darkroom Cookboook. Here, for instance: Darkroom Cookbook. That should set you on your way.
Good luck! Mixing your own film and paper developers can give you an unparalled amount of control over your development process. If you develop a lot of film, it can be cost effective too. And you get to learn cool stuff.
Freakscene
Obscure member
Indeed, the Darkroom Cookbook is the one to get.Thank you so much for the recommendation, I'm definitely ordering a copy!
titrisol
Bottom Feeder
Also, the 3rd Ed of the film developing cookbook came a few years ago, it is a great buy
Glenn2
Well-known
If Tom was still with us am sure he would have provided this link….
Toms developers
Toms developers
Share:
-
This site uses cookies to help personalise content, tailor your experience and to keep you logged in if you register.
By continuing to use this site, you are consenting to our use of cookies.